PAXLOVID - Pfizer Covid-19 Antiviral Treatment
A solution for both a prescription and medication
- Reduces severe COVID-19 and mortality rate by 90% when used within 5 days.
- 100% made by Pfizer
- Prescription by licensed doctor in the U.S
- Pick up at a pharmacy near you
PAXLOVID - Pfizer Covid-19 Antiviral Treatment
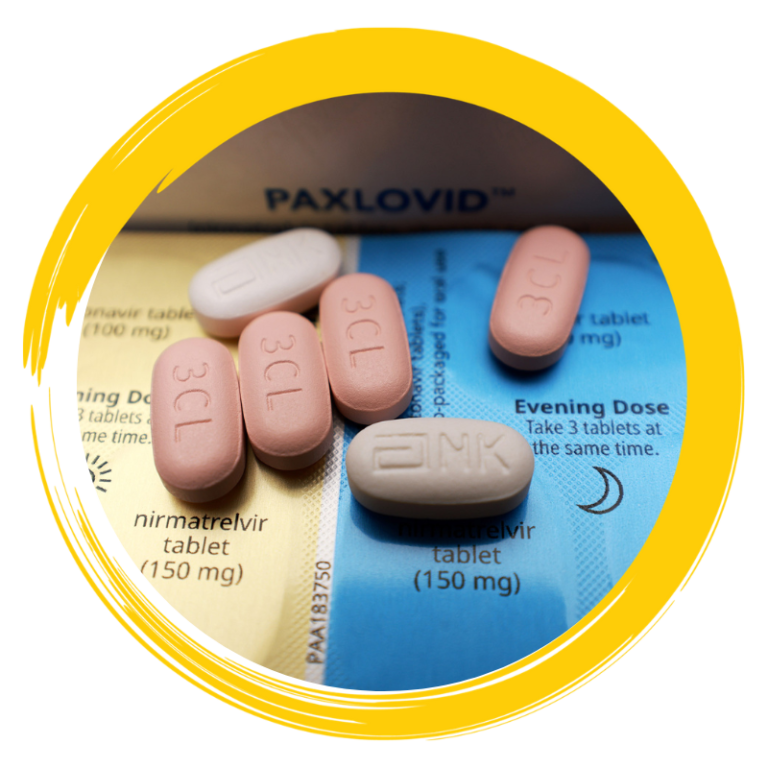
Paxlovid is the brand name of the COVID-19 oral medication, nirmatrelvir/ritonavir, developed by Pfizer in the United States, which has been granted emergency use authorization by the FDA for the treatment of COVID-19. Paxlovid is approved for use in treating mild to moderate COVID-19 in patients aged 12 and above who are at high risk of developing severe illness. Taking Paxlovid within 5 days of symptom onset can significantly reduce the risk of severe COVID-19 and mortality by around 90%.

How to take PAXLOVID
- AXLOVID 由 2 种药物组成:nirmatrelvir 片剂和 ritonavir 片剂。 2种药一起服用,每天2次,连服5天
- Nirmatrelvir is an oval, pink tablet
- Ritonavir is a white or off-white tablet
- PAXLOVID is available in 2 Dose Packs. Your healthcare provider will prescribe the PAXLOVID Dose Pack that is right for you
- If you have kidney disease, your healthcare provider may prescribe a lower dose. Talk to your healthcare provider to make sure you receive the correct dose pack
- Do not remove your PAXLOVID tablets from the blister card before you are ready to take your dose
- Take your first dose of PAXLOVID in the Morning and Evening, depending on when you pick up your prescrpition, or as recommended by your healthcare provider
- Swallow the tablets whole. Do not chew, break, or crush the tablets
- Take PAXLOVID with or without food
- Do not stop taking PAXLOVID without talking to your healthcare provider, even if you feel better
- If you are taking a ritonavir- or cobicistat-containing medicine to treat hepatitis C or human immunodeficiency virus (HIV), you should continue to take your medicine as prescribed by your healthcare provider


Possbile side effects for PAXLOVID
Allergic Reactions. Allergic reactions, including severe allergic reactions (known as ‘anaphylaxis’), can happen in people taking PAXLOVID, even after only 1 dose. Stop taking PAXLOVID and call your healthcare provider right away if you get any of the following symptoms of an allergic reaction:
- hives
- trouble swallowing or breathing
- swelling of the mouth, lips, or face
- throat tightness
- hoarseness
- skin rash
Liver problems: Tell your healthcare provider right away if you have any of these signs and symptoms of liver problems: loss of appetite, yellowing of your skin and the whites your eyes (jaundice), dark-colored urine, pale-colored stools and itchy skin, or stomach area (abdominal) pain.
Resistance to HIV medicines: If you have untreated HIV infection, PAXLOVID may lead to some HIV medicines not working as well in the future.
Other possible side effects include: altered sense of taste, diarrhea, high blood pressure, muscle aches, abdominal pain, nausea, feeling generally unwell.
Allergic Reactions. Allergic reactions, including severe allergic reactions (known as ‘anaphylaxis’), can happen in people taking PAXLOVID, even after only 1 dose. Stop taking PAXLOVID and call your healthcare provider right away if you get any of the following symptoms of an allergic reaction:
- hives
- trouble swallowing or breathing
- swelling of the mouth, lips, or face
- throat tightness
- hoarseness
- skin rash
Liver problems: Tell your healthcare provider right away if you have any of these signs and symptoms of liver problems: loss of appetite, yellowing of your skin and the whites your eyes (jaundice), dark-colored urine, pale-colored stools and itchy skin, or stomach area (abdominal) pain.
Resistance to HIV medicines: If you have untreated HIV infection, PAXLOVID may lead to some HIV medicines not working as well in the future.
Other possible side effects include: altered sense of taste, diarrhea, high blood pressure, muscle aches, abdominal pain, nausea, feeling generally unwell.
These are not all the possible side effects of PAXLOVID. Not many people have taken PAXLOVID. Serious and unexpected side effects may happen. PAXLOVID is still being studied, so it is possible that all of the risks are not known at this time.
Report side effects or problems with the appearance or packaging of PAXLOVID to FDA MedWatch at www.fda.gov/medwatch or call 1-800-FDA-1088 or you can report side effects to Pfizer Inc. at the contact information provided below.
FAQs
Paxlovid is for adults and children 12 and older weighing at least 40 kg (88.2 lbs) who are at higher risk for developing serious COVID-19 disease that may lead to hospitalization and/or death. Paxlovid should be considered for patients who meet the following criteria:
- Test positive for SARS-CoV-2 (with PCR or antigen test, including at-home tests), AND
- Are able to begin treatment as soon as possible after diagnosis of COVID-19 and within 5 days of symptom onset, AND
- Have symptoms consistent with mild-to-moderate COVID-19, AND
- Have one or more risk factors for severe COVID
The benefit of a 5-day treatment course of Paxlovid was demonstrated in the clinical trial that supported the EUA. This study showed that among non-hospitalized, unvaccinated patients at high risk of progression to severe disease, treatment with Paxlovid reduced the risk of hospitalization or death by 88%.
Observational data, including vaccinated patients, from Israel1, United States2, and Hong Kong3 is consistent with benefit in high-risk patients:
- 67% reduction in hospitalizations and 81% reduction in deaths compared to the untreated for patients over 65
- 45% reduction in hospitalization and greater reductions for obese or unvaccinated patients among adult patients
- 75% reduction in death compared to non-users.
Do not take PAXLOVID if:
- You are allergic to nirmatrelvir, ritonavir, or any of the ingredients in PAXLOVID
- You are taking any of the following medicines:
- alfuzosin
- amiodarone
- apalutamide
- carbamazepine
- colchicine
- dihydroergotamine
- dronedarone
- eletriptan
- eplerenone
- ergotamine
- finerenone
- flecainide
- flibanserin
- ivabradine
- lomitapide
- lovastatin
- lumacaftor/ivacaftor
- lurasidone
- methylergonovine
- midazolam (oral)
- naloxegol
- phenobarbital
- phenytoin
- pimozide
- primidone
- propafenone
- quinidine
- ranolazine
- rifampin
- John’s Wort (hypericum perforatum)
- sildenafil (Revatio®) for pulmonary arterial hypertension
- silodosin
- simvastatin
- tolvaptan
- triazolam
- ubrogepant
- voclosporin
Taking PAXLOVID with these medicines may cause serious or life-threatening side effects or affect how PAXLOVID works.
These are not the only medicines that may cause serious side effects if taken with PAXLOVID. PAXLOVID may increase or decrease the levels of multiple other medicines. It is very important to tell your healthcare provider about all of the medicines you are taking because additional laboratory tests or changes in the dose of your other medicines may be necessary while you are taking PAXLOVID. Your healthcare provider may also tell you about specific symptoms to watch out for that may indicate that you need to stop or decrease the dose of some of your other medicines.
You can take any available FDA-authorized COVID-19 viral test including the at-home covid test kit (eg, RT-PCR, rapid antigen, etc) to determine if you have COVID-19.
eople of all ages or groups can be infected with COVID-19. Some people are more likely than others to get very sick from COVID-19, which can lead to hospitalization or death, even when symptoms start off as mild.
Some people are at a greater risk of COVID-19 becoming severe because they meet certain criteria, including where they live, work, or have difficulty accessing health care. This includes many people from racial and ethnic minority groups and people with disabilities.
In addition, having one or more of the following factors puts you at high risk of getting severe COVID-19*:
- 65 years or older
- Cancer
- Chronic kidney disease
- Chronic liver disease
- Chronic lung disease (including moderate-to-severe asthma, chronic obstructive pulmonary disease [[COPD]], emphysema, and chronic bronchitis)
- Cystic fibrosis
- Dementia or other neurological conditions
- Diabetes (Type 1 or Type 2)
- Disabilities (including attention-deficit/hyperactivity disorder [[ADHD]], cerebral palsy, birth defects, intellectual and developmental disabilities, learning disabilities, spinal cord injuries, Down syndrome)
- Heart conditions
- HIV infection
- Immunocompromised condition or weakened immune system
- Mental health conditions (including mood disorders, schizophrenia spectrum disorders, and depression)
- Overweight or having obesity
- Physical inactivity
- Pregnant or recently pregnant (for at least 42 days following end of pregnancy)
- Sickle cell disease or thalassemia
- Smoking, current or former
- Solid organ or blood stem cell transplant
- Stroke or cerebrovascular disease
- Substance use disorders (such as alcohol, opioid, or cocaine use disorder)
- Tuberculosis
- AXLOVID 由 2 种药物组成:nirmatrelvir 片剂和 ritonavir 片剂。 2种药一起服用,每天2次,连服5天
- Nirmatrelvir is an oval, pink tablet
- Ritonavir is a white or off-white tablet
- PAXLOVID is available in 2 Dose Packs. Your healthcare provider will prescribe the PAXLOVID Dose Pack that is right for you
- If you have kidney disease, your healthcare provider may prescribe a lower dose. Talk to your healthcare provider to make sure you receive the correct dose pack
- Do not remove your PAXLOVID tablets from the blister card before you are ready to take your dose
- Take your first dose of PAXLOVID in the Morning and Evening, depending on when you pick up your prescrpition, or as recommended by your healthcare provider
- Swallow the tablets whole. Do not chew, break, or crush the tablets
- Take PAXLOVID with or without food
- Do not stop taking PAXLOVID without talking to your healthcare provider, even if you feel better
- If you are taking a ritonavir- or cobicistat-containing medicine to treat hepatitis C or human immunodeficiency virus (HIV), you should continue to take your medicine as prescribed by your healthcare provider
Talk to your healthcare provider if you do not feel better or if you feel worse after 5 days.
If you miss a dose of PAXLOVID within 8 hours of the time it is usually taken, take it as soon as you remember. If you miss a dose by more than 8 hours, skip the missed dose and take the next dose at your regular time. Do not take 2 doses of PAXLOVID at the same time.
If you take too much PAXLOVID, call your healthcare provider or go to the nearest hospital emergency room right away.
The 5-day treatment course of PAXLOVID should be initiated as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Allergic Reactions. Allergic reactions, including severe allergic reactions (known as ‘anaphylaxis’), can happen in people taking PAXLOVID, even after only 1 dose. Stop taking PAXLOVID and call your healthcare provider right away if you get any of the following symptoms of an allergic reaction:
- hives
- trouble swallowing or breathing
- swelling of the mouth, lips, or face
- throat tightness
- hoarseness
- skin rash
Liver problems: Tell your healthcare provider right away if you have any of these signs and symptoms of liver problems: loss of appetite, yellowing of your skin and the whites your eyes (jaundice), dark-colored urine, pale-colored stools and itchy skin, or stomach area (abdominal) pain.
Resistance to HIV medicines: If you have untreated HIV infection, PAXLOVID may lead to some HIV medicines not working as well in the future.
Other possible side effects include: altered sense of taste, diarrhea, high blood pressure, muscle aches, abdominal pain, nausea, feeling generally unwell.
These are not all the possible side effects of PAXLOVID. Not many people have taken PAXLOVID. Serious and unexpected side effects may happen. PAXLOVID is still being studied, so it is possible that all of the risks are not known at this time.
Report side effects or problems with the appearance or packaging of PAXLOVID to FDA MedWatch at www.fda.gov/medwatch or call 1-800-FDA-1088 or you can report side effects to Pfizer Inc. at the contact information provided below.
How Mojoon Works
The Mojoon telemedicine platform is encrypted with HIPAA security levels to ensure the confidentiality and security of your information. The online medical questionnaire has been confirmed by our team of doctors and is designed to obtain the necessary information for diagnosis.

Register and complete the questionnaire
Complete your personal information and complete the questionnaire to the best of your knowledge
Choose your treatment plan and make payment
Choose the treatment plan that suits you. Our pricing is transparent and open and in most of the cases are lower than most insurance Co-Pays.
Doctor Review
Licensed doctors will review prescriptions within 24 hours and contact patients through our internal messaging system.
Free delivery to your doorstep or pick up at a local pharmacy.
Free and discreet delivery of medication to your doorstep or pick up at a partnered local pharmacy, ensuring your privacy and anonymity.
Real User Experiences
It was truly a lifesaver. After being diagnosed with COVID-19, I felt really uncomfortable. I had an online consultation the next afternoon and received a prescription. The Pfizer medication was delivered the next day in the afternoon. It was so convenient! Thank you Mojoon!
Michelle from San Diego ClientMy parents got COVID-19 shortly after they arrived in the US without any insurance and couldn't communicate well in English. Thanks to Mojoon, they were able to get COVID-19 medication quickly after being diagnosed, and we felt much more relieved. Mojoon is truly convenient, and I highly recommend it to everyone. Five stars!
Charles from Boston ClientAll the local pharmacies near us were sold out of Paxlovid, so we had no choice but to try Mojoon. Surprisingly, the medication was delivered the next day via FedEx overnight shipping. It was very convenient, and we are grateful for the service.
Chris from Houston Client
Mojoon's online Chinese consultation service is truly convenient and fast! Their customer service is also in Chinese, and they can usually respond to questions by text message or email on the same day. I obtained a prescription for COVID-19 medication through the consultation on January 5th, and received the medication by noon on the 7th. 👍🏻
Mike From NYC Client